CESP expands in Poland and Greece
Latest news from Poland and Greece for CESP
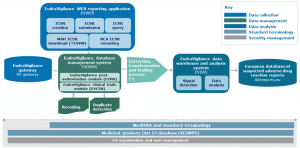
From 3rd April 2017 it will be possible to submit the following procedures via Common European Submission Portal (CESP) to Poland:
- Initial Marketing Authorisation Applications (MAAs)
- Variations
- Renewals
The submissions may concern national and European procedures (MRP, DCP) both for human and veterinary medicinal products.
Also from 3rd April 2017 the following submissions should be transmitted through CESP for Greece:
- All submissions for Mutual Recognition/Decentralised Procedures (MRP/DCP)
- national procedures relating to approvals, variations, renewals, PSURs, ASMFs etc., as well as any other documentation should be submitted using CESP.
- Responses to deficiencies and any other additional information that can be requested from the EOF within the claims of assessment.
What does this mean for you?
To date in Poland, only submissions concerning initial MAAs were possible via CESP. There are still some national requirements in Poland, however. All documents that require an original signature can be either submitted
- in electronic version with a valid electronic signature or
- must be submitted in parallel to CESP in paper.
- Documentation once submitted via CESP should be submitted via this channel throughout its whole lifecycle.
For submissions into Greece, additional CD/DVDs are no longer required. In addition, for each submission, a corresponding reference number will be automatically assigned and will be sent directly to the applicant.
Where can I find more information?
Full details are available on the Polish Ministry’s website (in Polish) here.
Full information is for Greece (in Greek) here.
We can help…
Ivowen are fully equipped to prepare and submit any applications on your behalf. Please contact us or our EuDRAcon partners for more information and for support of your dossier compilation or updates.
Written by Majella Ryan